0 Views· 08 August 2023· Education
Molarity Dilution Problems Solution Stoichiometry Grams, Moles, Liters Volume Calculations Chemistry
This chemistry video tutorial focuses on molarity and dilution problems. It shows you how to convert between molarity, grams, moles, and liters. It's very useful for students learning solution stoichiometry. This video contains plenty of notes, examples, equations / formulas and practice problems.
General Chemistry 1 Review:
https://bit.ly/3DNmZqb
PDF Worksheet - 160 Questions:
https://bit.ly/37SVLn6
Here is a list of topics:
1. How to calculate the Molarity of the solution given grams, moles, volume in ml or liters.
2. Determining the mass given the concentration in molarity and the volume in milliliters.
3. Using unit conversion / dimensional analysis to calculate the volume of the solution in mL.
4. Solute, Solvent, Solution Relationship
5. How to increase and decrease the concentration of a solution by adding water or removing water through dilution or evaporation.
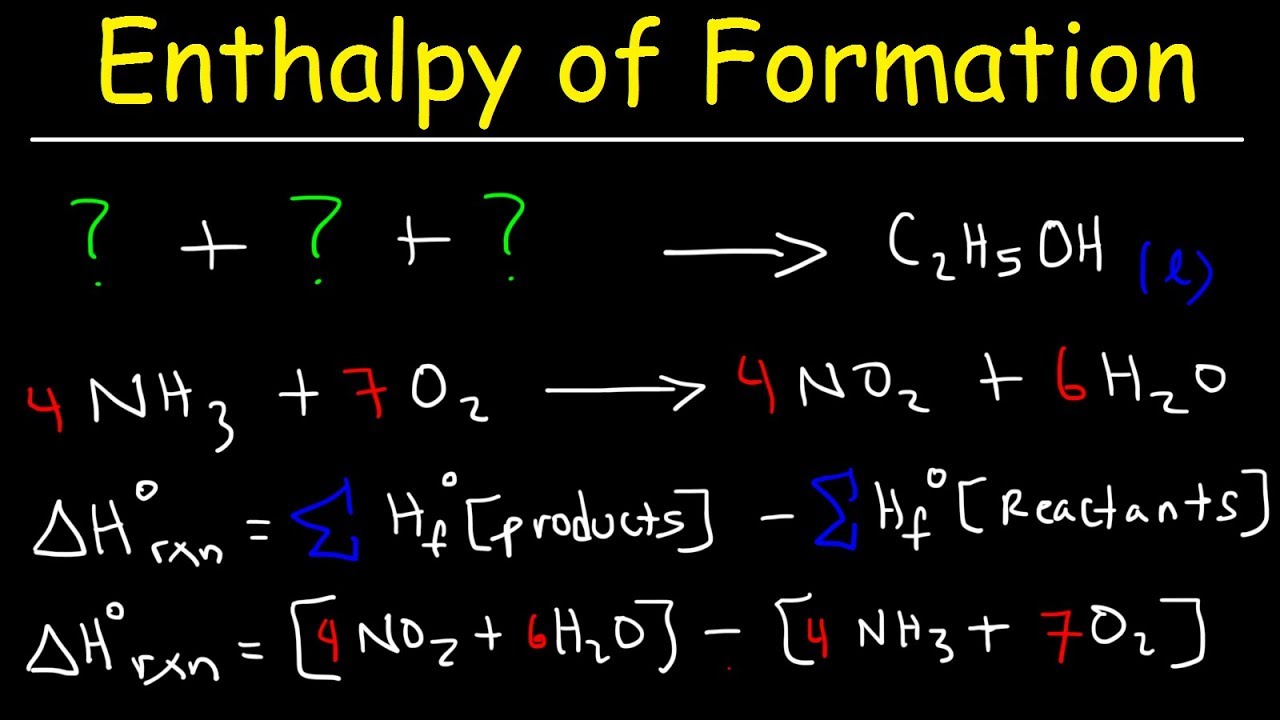
6. Molarity and Dilution Problems - M1V1=M2V2
7. Solution Stoichiometry - Actual Yield, Theoretical Yield, and Percent Yield
8. Molarity Stoichiometry - Limiting and Excess Reactant
9. How To Find The Amount of Excess Reactant Remaining / Left Over in Volume in mL, Mass in grams, and moles
10. Single Replacement Reactions - Metal and Halide Displacement - Activity Series
11. Predicting the products of a double replacement reaction - acid base neutralization and precipitation reactions
12. How to calculate the maximum amount of product / theoretical yield given molarity, volume, and grams of the reactants
13. Basic Dilution Calculations
14. Using the Dilution Formula / Equation M1V1=M2V2 to solve acid base titration problems and redox reaction titrations
15. Mixture Problems - Finding the Molarity after mixing two or more solutions









0 Comments