2 Views· 08 August 2023· Education
Ionization Energy - Basic Introduction
This chemistry video tutorial provides a basic introduction into Ionization Energy. It discusses the periodic trends and exceptions as well as providing plenty of examples and practice problems. The first ionization energy is the energy required to remove an electron from a gaseous atom or ion. The second ionization energy is associated with the removal of the second electron. Ionization energy increases with effective nuclear charge but decreases with distance, shielding and electron repulsions. Paired electrons typically have lower ionization energies that unpaired electrons. This explains how to determine which element and ion has the greater first ionization energy. It covers cations and anions and how to rank elements in order of increasing ionization energy. In addition, it discusses how to identify the element given the ionization energies of that element using valence electrons and core electrons.
New Chemistry Video Playlist:
https://www.youtube.com/watch?v=bka20Q9TN6M&t=25s&list=PL0o_zxa4K1BWziAvOKdqsMFSB_MyyLAqS&index=1
Access to Premium Videos:
https://www.patreon.com/MathScienceTutor
Facebook: https://www.facebook.com/MathScienceTutoring/








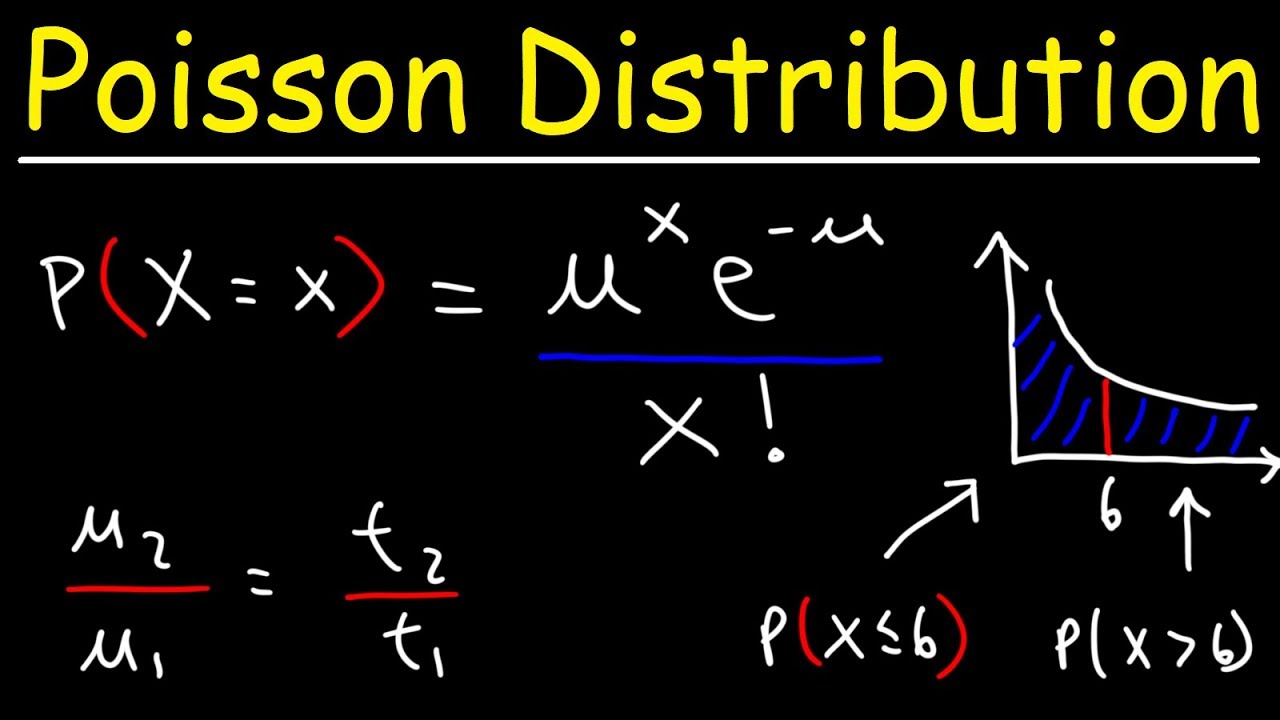
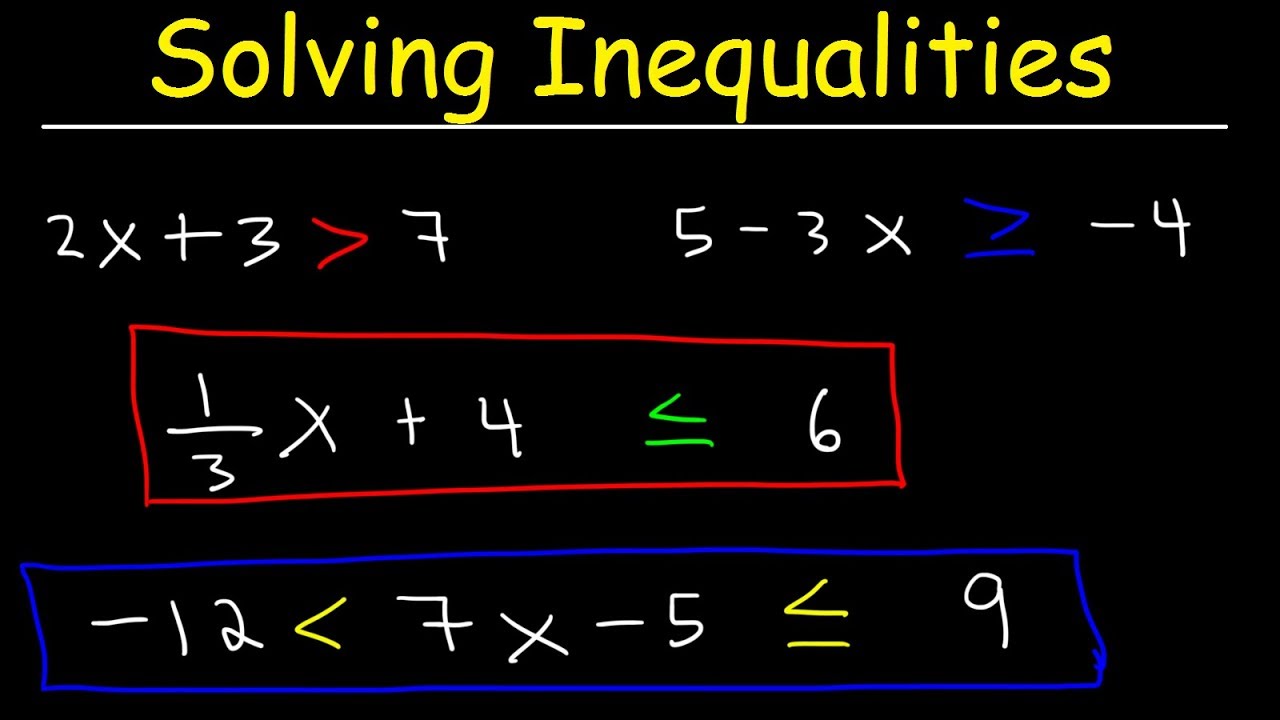
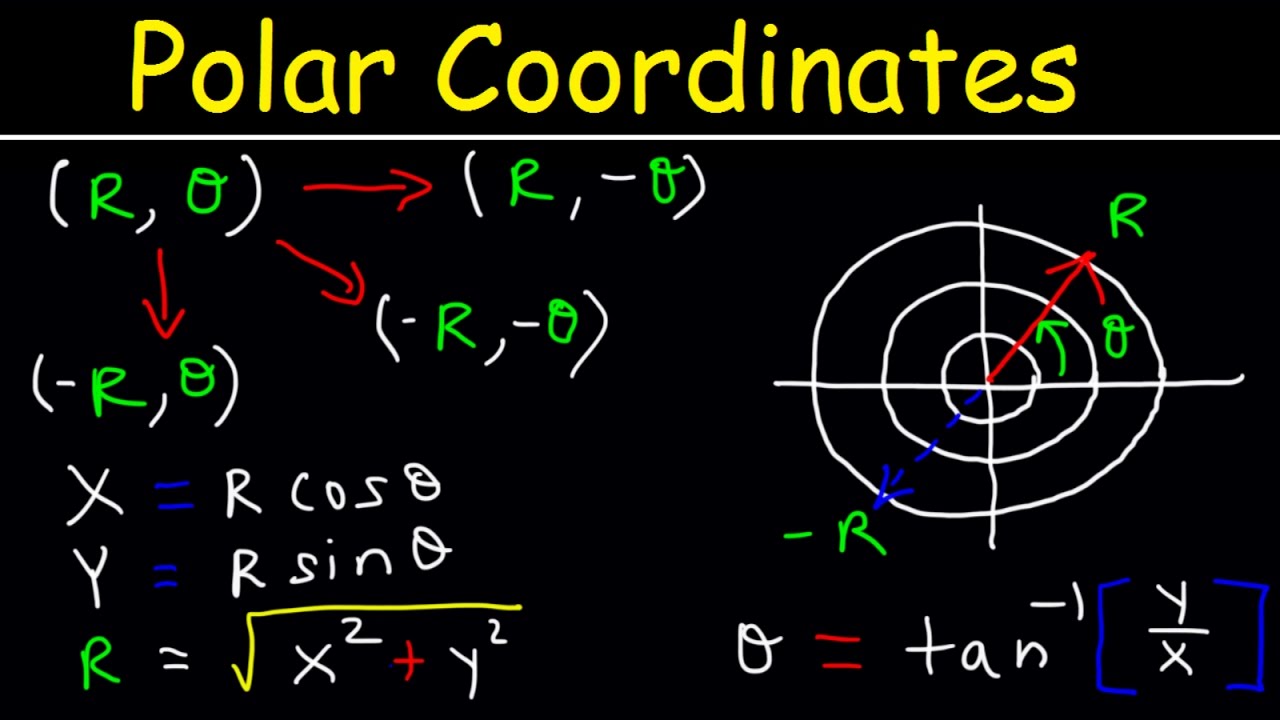


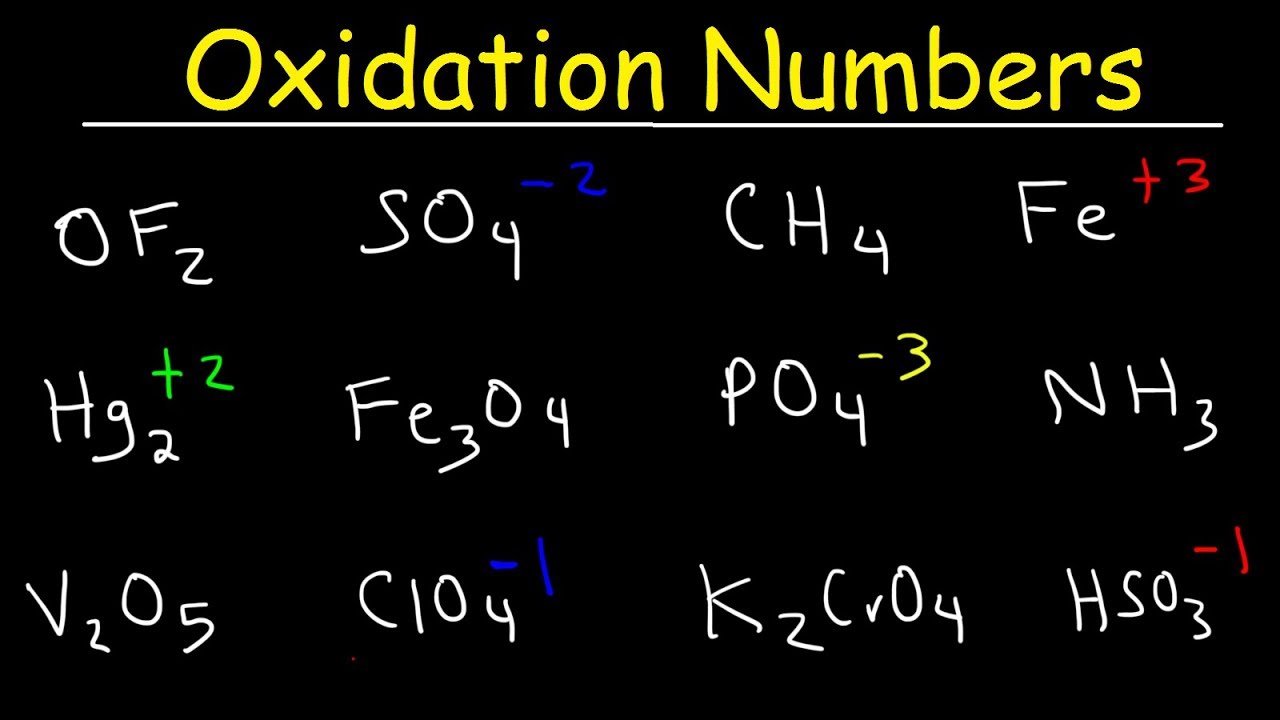


0 Comments